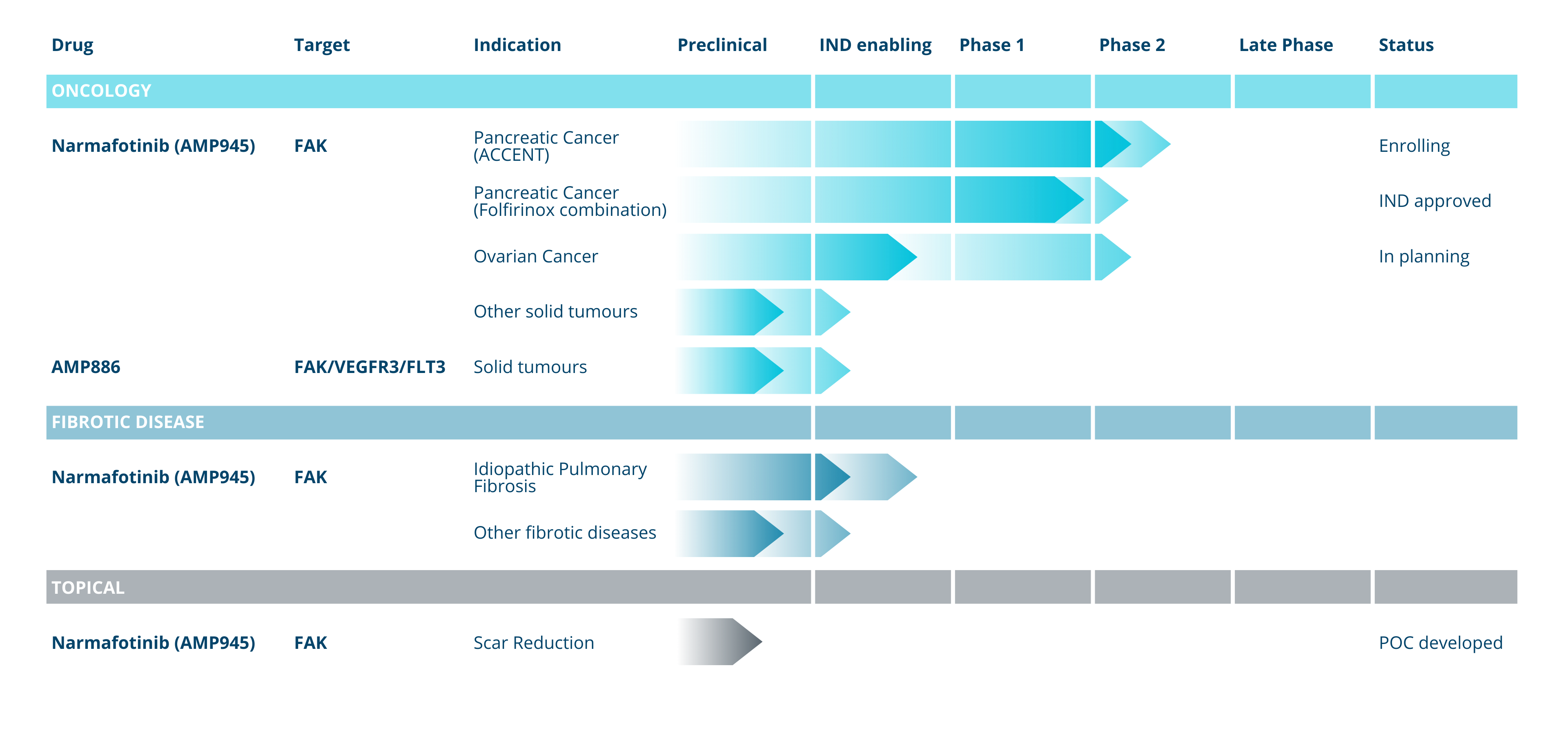
Pipeline
Our Technology
Amplia Therapeutics is currently focused on the clinical development of two highly-promising drug candidates targeting Focal Adhesion Kinase (FAK).
FAK inhibitors have been described as a ‘heavy punch’ to cancer, showing promising potential when used in combination with existing ‘standard of care’ therapies in the treatment of solid cancers. Our FAK inhibitors affect both the cancer cells themselves and the surrounding tissue, making tumours more vulnerable and responsive to currently used, and developmental, treatments.
Oncology
Focal Adhesion Kinase (FAK) is upregulated in many cancers where it supports cell survival and drives cell growth and migration. In addition, it is directly involved in several important biological processes in the tumour microenvironment i.e. the region surrounding the tumour; including the formation of new fibrotic tissue and generation of an immunosuppressed environment. When cancers become established in the body, they often use these processes to improve their own survival. Amplia’s Cancer Program is directed at using its FAK inhibitors to block FAK activity in the cancer cells and the surrounding tissue, thereby blocking the above processes and making the tumours more susceptible to chemotherapy.
Amplia’s lead drug candidate, narmafotinib, is a highly selective and potent FAK inhibitor that has shown promising results in preclinical studies for the treatment of pancreatic and ovarian cancers.
Pancreatic Cancer: Pancreatic cancer is a difficult to treat cancer, with a low survival rate, typically detected late in disease progression. There were an estimated 4,500 diagnoses in Australia in 2023 and the estimated 5 year survival rate is 13%. Amplia has demonstrated the efficacy of narmafotinib in preclinical models of pancreatic cancer and is now in phase 2 clinical trial in advanced pancreatic cancer in Australia and South Korea.
Amplia has orphan drug designation and an open IND for a trial in pancreatic cancer from the US FDA.
Ovarian: Over 315,000 women develop ovarian cancer annually and high grade serous ovarian cancer (HGSOC) accounts for ≥90% of cases. HGSOC has poor prognosis with a 10-year survival rate of 15%. Platinum-based chemotherapy is the standard-of-care (SOC) treatment, however, nearly all recurrent HGSOC develop platinum-resistance limiting treatment options.
Fibrosis
Fibrosis is the formation of excessive fibrous connective tissues that can result from chronic inflammation, chronic injury or disease. Fibrosis occurs in many different diseases and it has been estimated that dysregulated fibrosis results in 45% of all deaths. Excessive fibrous tissue can impair the normal functioning of various organs in the body, such as the lungs, liver, heart and kidneys, resulting in major health issues.
Focal Adhesion Kinase (FAK) plays an important role in fibrotic disease. Amplia’s FAK inhibitors have been shown to block fibrosis in established models of both idiopathic pulmonary fibrosis (IPF) and liver fibrosis (NASH).
Amplia has orphan drug designation for IPF from the US FDA.


